Radiation in Everyday Life
Part 1: The Concept of Radiation
The word Radiation conjures up different images and ideas in different people. But what exactly is radiation?
Radiation is essentially energy travelling through a medium such as air, water, or space.

Part 2: Categories of Radiation
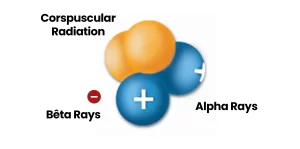
Radiation can be divided into two categories: electromagnetic radiation, which travels in the form of waves, and corpuscular radiation (more commonly known as particle radiation), which travels in the form of particles.
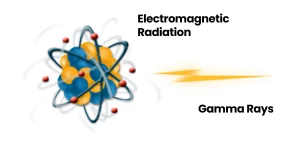
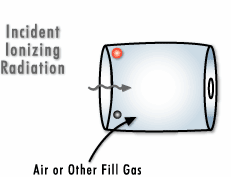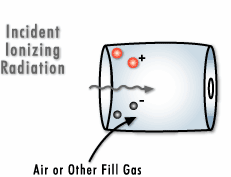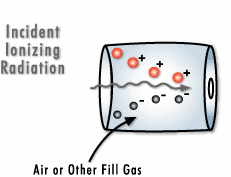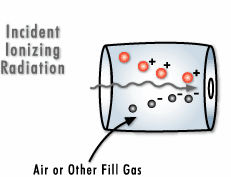
Electromagnetic radiation is also referred to as photonic radiation. Photons may transport energy that is strong enough to draw electrons away from the atoms through which the radiation passes.
This form of radiation can be qualified as “ionizing radiation.” Gamma rays are a type of electromagnetic radiation. They are essentially identical to X-rays, differing only in their source and the amount energy they produce.
To create a Geiger counter
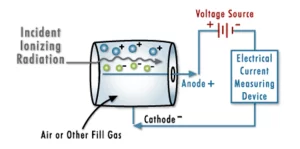
Particle radiation is radiation composed of real particles, as opposed to waves. These particles interact with the matter they come into contact with.
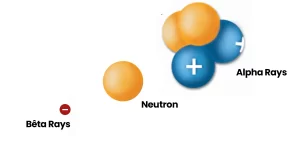
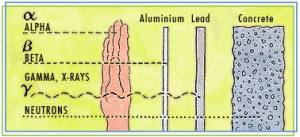
Alpha particles, neutrons and beta particles are examples of ionizing radiation.
Alpha Rays
Alpha rays are helium nuclei, consisting of large particles with two charges. Due to their significant ionization power, they are used in smoke detectors and devices for static elimination.
Neutrons
Neutron radiation plays a crucial role in nuclear fission. It is also used in practical applications such as soil humidity measurements for highway paving.
Beta Rays
Beta rays are either electrons (with a negative charge) or positrons (with a positive charge). They are utilized in biological tracer studies to observe and understand different biological processes.
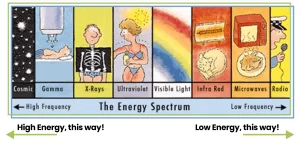
Experts typically start by identifying different types of electromagnetic radiation. X-rays, cellular phone signals, and UV radiation from the sun all consist of photons, which are energy packets that can be described as electromagnetic waves with varying energies and frequencies.
The electromagnetic spectrum illustrates electromagnetic radiation according to frequency. The greater the frequency, the greater the radiation energy.
Although these forms of electromagnetic radiation are identical in nature, their effects on the human body are quite different.
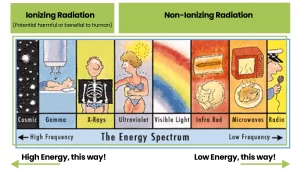
Based on these effects, X-rays are called “ionizing radiation”, that is, they produce ions when they strike a target.
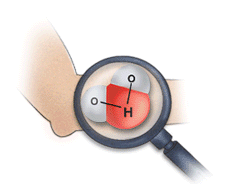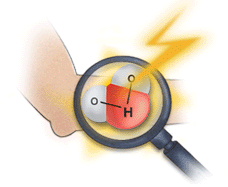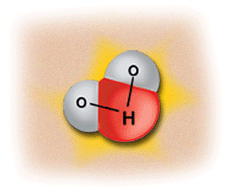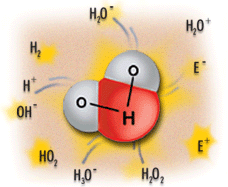
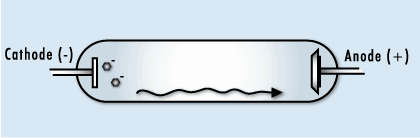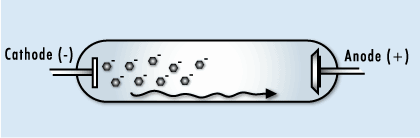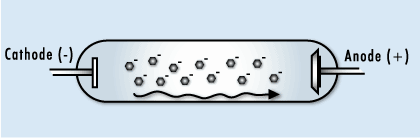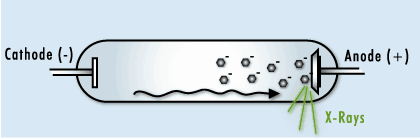
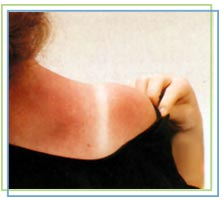
This image shows the human body being exposed to X-rays.
This is only one of many interactions occurring with ionizing radiation. The X-rays ionize the water molecules (H2O) that are part of each cell in the body, and cause the buildup of free radicals, which are unstable, charged particles and ions.
Most of the time, the ions will quickly recombine back into the original H2O molecule. But in some cases, they may recombine to form undesirable molecules, like hydrogen peroxide (H2O2), within the cell and the cell may die if it can’t cope with the buildup of peroxide.
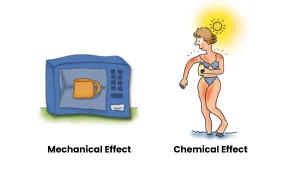
In contrast, radiation from lasers and microwaves, as well as from UV rays and radio frequencies is considered “non-ionizing” radiation.
Since these photons have lower energy, they cannot ionize atoms, and the effects on the human body are either mechanical (like heating a cup of water in a microwave oven) or chemical (like getting a sunburn).
Part 3: Sources of Radiation
Radiation comes either from radiation emitting devices such as X-ray machines or microwave ovens, or from radiation emitting processes like radioactive decay from radioisotopes or fusion reaction in the Sun.
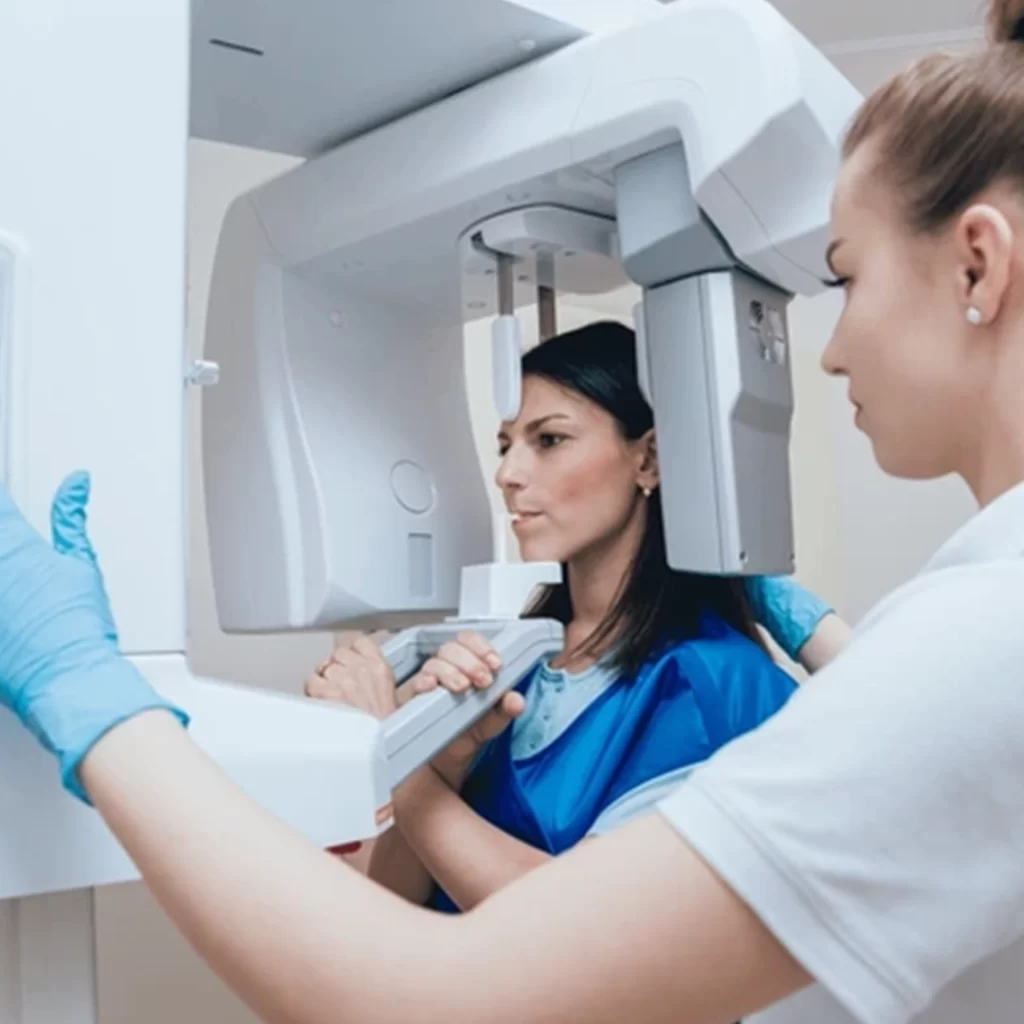
Dental X-ray
Regularly used in dental offices, this device accelerates electrons toward a high-density target to generate X-rays.

The Sun
The Sun is a natural fusion reactor. In its core, Hydrogen atoms fuse to generate heat (infrared radiation) and all other types of radiation, including UV radiation.
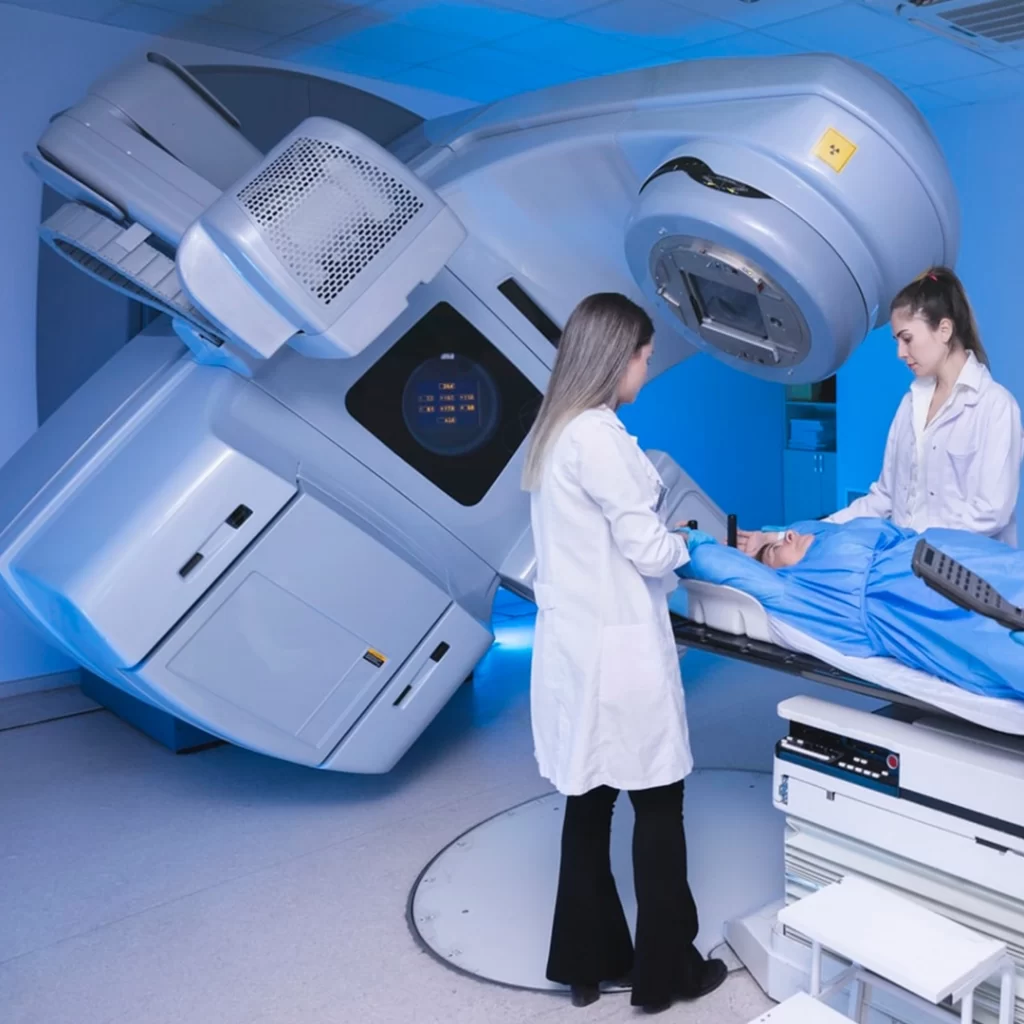
Linear Accelerator (LINEAC)
This device is the weapon of choice in fighting tumours. It is used in radiation oncology to kill cancerous cells by accelerating heavy particles.

Commercial Radioisotopes
This is a very common use of radioactive material. Most of the time, radioisotopes are created by bombarding a target with neutron rays or heavy nuclei in a reactor or accelerator.
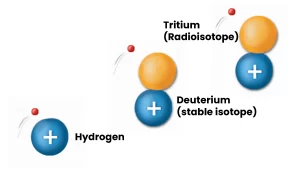
The information presented here focuses on radioisotopes. In nature, elements have different versions, known as isotopes, which are like “cousins” with the same number of protons but varying numbers of neutrons. When these isotopes are unstable, they become radioisotopes.
Radioactive isotopes, also known as radioisotopes, exist all around us naturally, and can even be found in elements such as carbon, hydrogen, and cobalt.
They can be found in the ground, for example, in the form of Uranium and its decay products such as radon, in equilibrium with all living things in the form of Carbon-14, and directly in the human body in the form of Potassium-40.
These would be examples of Naturally Occurring Radioactive Material, referred to as NORM.
Part 4: Radiation Protection Basics
There is a wide variety of sources and applications of radiation. Depending on the nature of the radiation, humans use different methods to protect themselves from exposure.
The science of radiation protection, also known as Health Physics, emerged in the public eye as radiation began to be widely used for a variety of applications – some practical, others innovative.

The main objective of Health Physics is to protect individuals and population groups against harmful effects of ionizing and non-ionizing radiation. The Canadian Radiation Protection Association is comprised of more than 300 members who study, work or are in the business of Radiation Protection and related fields.
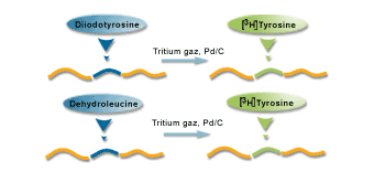
Labelling
Radioactive materials are used as tracers to mark a molecule or a compound that may become a future treatment or medicine.

Gauging
In many industries, including paper mills, mines and packaging plants, a radioactive source can be used to calculate the level of a liquid in a container, or to evaluate the density of a material.
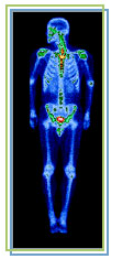
Imaging
In nuclear medicine, a radioactive solution is injected into the body to evaluate the functioning of organs, and to take real-time images.
You are probably already familiar with ways in which humans protect themselves from exposure to radiation.
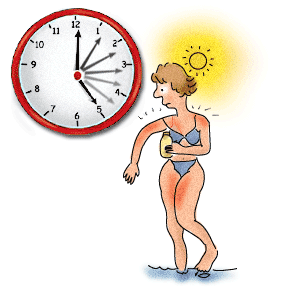
For example, how to protect yourself from the harmful UV rays from the sun.
Shielding
An example of this would be applying sunscreen (specialists call this “shielding”).
Lead can also be used as a shield from X-rays and gamma rays.
Some sources of radiation are not very penetrative, and a piece of plastic would provide adequate protection, yet other sources are so minute that even a certain thickness of air will provide a shield.
Minimize exposure
For example, reduce the amount of time you spend in the sun.
Distance
As examples, X-ray technicians rarely stay close to a patient when they are taking X-rays, and those who use radioisotopes use remote handling tools when there are high levels of radioactivity.
To summarize, the most effective ways to protect yourself from exposure are adequate shielding, exposure reduction, and increasing the distance from the radioactive source.

How is exposure to radiation measured?
Exposure to radiation is measured in millisieverts (mSv).
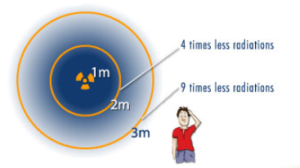
The “inverse square law” is often used to calculate exposure. At 1 metre, you receive a specific amount of radiation, at 2 metres, 4 times less, and at 3 metres, 9 times less. The amount of exposure depends on the strength of the radioactive source.
In Canada, the amount of natural background radiation you receive each year is between 2 and 4 mSv.
For more information on radiation and radiation safety, visit the following page:
or
Contact Us with your questions
Originally Created By:
Stéphane Jean-François, Marie Archambault & Geneviève Corriveau
Some images were used with permission from:
Dr. Ian Hore-Lacy, Uranium Information Centre, Australia
Dr. Paul Frame, Oakridge Associated Universities





